Spectroscopy - Some Practicalities
How do we make excited atoms?
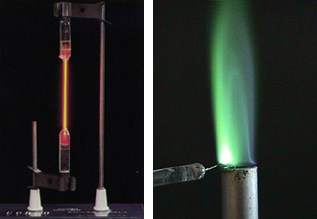
Exciting an atom requires the input of energy. Two of the most common ways of supplying this energy are with an electric potential or a flame. In a discharge tube (below left) a high voltage is placed across or through a gas or piece of the element. In a flame (below right) a piece of nichrome wire is generally used to hold a compound that contains the element. This wire is then held in the flame. The color we see in each case is the color of the light emitted when the atoms return to the ground state.
How do we get individual lines in the spectrum?
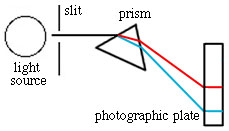
The excited atoms returning to the ground state are emitting light of various wavelengths in all directions, yet, in the spectra we have seen there are individual lines. To accomplish this, the emitted light must be focused and then separated into its constituent wavelengths (called dispersion). A narrow slit and prism serve this purpose (see below). The slit screens out all but a narrow beam of light. This light passes through a prism and separates due to the wavelength dependence of refractive index. A grating, which separates the light by reflection and interference, is now more commonly used in place of the prism.
What does a spectrometer look like?
The simplest spectrometer, or spectroscope, is composed of a slit, grating and scale in a housing.
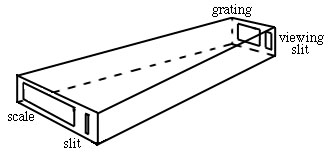
The light comes in the slit from the excited atoms, is dispersed, and viewed on a scale with your eye.
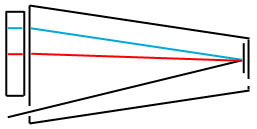
The spectra we have seen so far were likely taken by replacing the scale with a piece of photographic film. More complex instruments replace the film with a photosensitive detector and provide a mechanism by which different wavelengths of light can be looked at individually.
This concludes the practicalities portion of the experiment. You may begin the experiment.








