Organic Shorthand
To simplify the process of drawing molecular structures chemists have developed a shorthand method. In this method, carbons are assumed to be present at any corner (point) in the drawing. It is also assumed that the carbon has as many hydrogens bonded to it as are required to complete its octet.
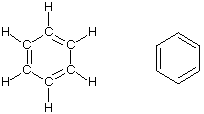
Benzene is a good example. The struture at left can be converted to the shorthand notation shown on the right.
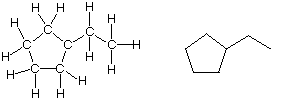
Ethylcycloentane is another good example. The struture at left can be converted to the shorthand notation shown on the right.
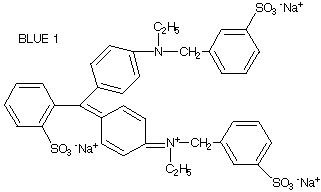
Being able to use shorthand to represent molecules becomes very important when dealing with big molecules. The complete structure of the dye Blue 1 is shown below.

Pretty confusing. The shorthand version is much simpler.